Abstract
Background Atrial fibrillation (AF) affects more than 30 million people and is the most common form of arrhythmia worldwide. Patients with cancer frequently develop AF during active treatment, and several studies have shown that AF is also prevalent at the time of cancer diagnosis. Patients with cancer and AF commonly require anticoagulant therapy (direct oral anticoagulants (DOAC) or vitamin K antagonist (VKA)) for stroke and systemic embolism (SE) prevention. Data on the efficacy and safety of oral anticoagulants are unclear in this patient population.
Objectives We sought to assess the rates of of stroke/SE and major bleeding in patients with cancer and AF on oral anticoagulant therapy (DOAC or VKA).
Methods A systematic search of MEDLINE, EMBASE, COCHRANE and all Evidence Based Medicine database (from inception to January 2022) were conducted. The primary efficacy outcome was stroke/SE, and the primary safety outcome was major bleeding as defined by the individual studies. The quality of the studies was assessed by the ROBINS-1 tool. Incidence rates of stroke/SE and major bleeding by anticoagulant therapies were pooled using random effects model and expressed as event per 100 patient-years with its associated 95% confidence intervals (CI) using R software (version 4.0.3).
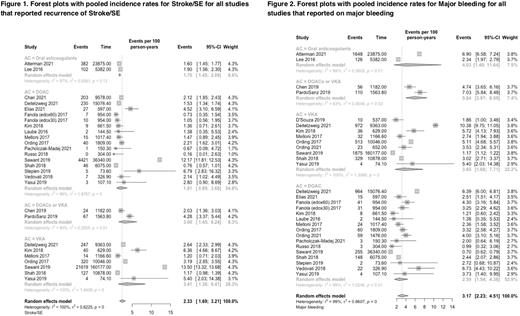
Results Of the total 2,151 article records that were screened, 24 observational studies (N=352,672 patients) from 12 different countries were included in the meta-analysis. The mean age of included patients was 73.4 years old, 70.8% of them were male, and the most represented race were caucasian and asian. The most common tumor sites were urinary tract tumors (N=16,865), followed by prostate (N=14,800), breast (N=14,451), gastrointestinal tract (N=13,897), hematological (N=11,697) and lung (N=9,278). All studies were judged to have moderate to serious risk of bias. Overall, the incidence rates of stroke/SE and major bleeding for patients with cancer and AF on oral anticogulant therapy were 2.33 (95% CI: 1.69 to 3.21) and 3.17 (95%CI: 2.23 to 4.51) per 100 person-years, respectively. The incidence of stroke/SE was 1.81 (95% CI: 0.89 to 3.68) per 100 patient-years for patients receiving a DOAC and 3.41 (95% CI: 1.38 to 8.41) for patients receiving VKA (Figure 1). Similarly, the incidence of major bleeding was 2.59 (95%CI: 1.54 to 4.38) per 100 patient-years for patients receiving a DOAC and 3.60 (95% CI: 1.68 to 7.71) for patients receiving VKA.
Conclusion Patients with cancer and AF receiving oral anticoagulant therapy are at high risk of both stroke/SE and major bleeding complications. DOACs seem to provide a good risk benefit profile for this patient population. Randomized controlled trials are needed to confirm these findings.
Disclosures
Wang:Servier: Other: Advisory board; Leo Pharma: Research Funding; Valeo: Other: Advisory board. Carrier:Sanofi: Consultancy; Servier: Consultancy, Honoraria; Bayer: Consultancy, Honoraria; BMS: Consultancy, Honoraria, Research Funding; Pfizer: Consultancy, Honoraria, Research Funding; Leo Pharma: Consultancy, Honoraria, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal